SUMMER 2020
Last day to withdraw was: 7/20/2020
| Friday July 24, 2020 (Only one part) Reaction Mechanisms II |
|
| Textbook references 14.6: Reaction Mechanisms |
Course Lectures 6.2 pdf Video Type 2 Reaction Mechanisms |
| Objectives 1. Define the rate limiting step and identify rate limiting steps in real life 2. Identify the rate limiting step in type II reaction mechanisms 3. Combine elementary reactions to determine the net reaction for the mechanism 4. Identify reaction intermediates in the type II reaction mechanism 5. Describe why the "slow" step that preceeds the RLS is an equilibrium reaction. 6. Use the equilibrium rate relationship to eliminate reaction intermediates in the reaction rate equation. |
|
Type II reaction mechanisms
All reaction mechanisms contain steps that when combined produce a net reaction involving observable reactants and products. If one of the steps is slower than the others, it limits the speed with which the reaction can take place. The slowest step is referred to as the rate limiting step or RLS for short. In yesterday's material, the Type I reaction mechanism was introduced and today we'll look at the type mechanism. The two mechanism differ in one important way. While Type I mechanisms always begin with the rate limiting step, Type II mechanisms has the RLS following a fast equilibrium step.In other words, the RLS isn't the first step. It's important to understand why the equilibrium step that precedes the RLS happens at all. Consider the following simple Type II mechanism: Step 1 A ↔ B (fast) Step 2 B + C ⟶ D (Slow) Step 1 generates it's product B very quickly; the idea being that Step 2 can use B (& C) to make product (D). The problem is that Step 2 is very slow and consequently can't use B as quickly as Step 1 produces it. So, B levels begin to rise and as they do, the Step 1 reverse reaction becomes important. At that point, Step 1 experiences both forward and reverse reactions. This, as we know is when equilibrium exists. |
|
A Type II reaction mechanism's rate equation is derived much the same way as a Type I rate equation. However, an additional step is necessary to eliminate reaction intermediates from the final result. Consider the following Type II mechanism: Step 1 A + B ↔ C (fast) Step 2 C + D ⟶ E (slow) We immediately identify the RLS as Step 2 and use it to write down the rate equation: rate = k [C] [D] problem However, reaction rate equations must not include reaction intermediates and "C" is a reaction intermediate that must be eliminated. To do this, we refer to the "fast" equilibrium step that occurs before the RLS in the mechanism. As it is an equilibrium step, we know that the rates of the forward and reverse reaction are equal: rateforward = ratereverse We also know that i. rateforward = kf [A] [B] and ii. ratereverse = kr [C] Combining these relationships we have: kf [A] [B] = rateforward = ratereverse = kr [C] or more simply ... kf [A] [B] = kr [C] Now, solve for [C] (kf / kr) [A] [B] = [C] And substitute this into the rate equation derived from the RLS: rate = k [C] [D] rate = k (kf / kr) [A] [B] [D] Grouping the constants k, kf and kr all together as a new constant km (mechanism) we get our final result: rate = km [A] [B] [D] Note that this rate equation contains no reaction intermediates |
|
Answer these questions 1. Consider the reaction mechanism below: Step 1 H2 ↔ 2H (fast) Step 2 H + CO ⟶ HCO (slow) Step 3 H + HCO ⟶ H2CO (fast) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? e. What is the overall reaction order? 2. Consider the reaction mechanism below: Step 1 Cl2 ↔ 2 Cl (fast) Step 2 Cl + CHCl3 ⟶ HCl + CCl3 (slow) Step 3 CCl3 + Cl ⟶ CCl4 (fast) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? e. What is the overall reaction order? Answers: Click and drag in the space below 1. a. H2 + CO ⟶ H2CO b. HCO and H c. Step 2 d. Rate = k [CO] [H2]½ e. overall reaction order is 3/2 2. a. CHCl3 + Cl2 ⟶ CCl4 + HCl b. Cl and CCl3 c. Step 2 d. Rate = k [CHCl3] [Cl2]½ e. overall reaction order is 3/2 |
|
| Thursday July 23, 2020 (Part 1) Integrated Rate Equations II |
|
| Textbook references 14.4: The Integrated Rate Law and how concentration depends on time |
Course Lectures 4.4 pdf Video Integrated Rate Equations 4.5 pdf Video Integrated Rate Equation Example |
| Objectives 1. Utilize graphical results to determine the reaction order. 2. Utilize graphical results to calculate the reaction rate constant. 3. Describe what is known as the "half life" for any physcial/chemical 4. Use half life formulations to determine half life and/or rxn rate constant. |
|
Integrated Rate Laws & Graphing The integrated rate laws can be graphed in ways to produce straight line behavior. At right, each of the three integrated rate law equations has been highlighted to identify the variables that should be graphed in search of a straight line plot. |
 |
| Typically, experimental rate data (concentration and time) is graphed all three ways: 0th order [A]t vs t 1st order ln[A]t vs t 2nd order 1/[A]t vs t
The graph that produces a straight line identifies the reaction order.
|
|
 |
|
| Answer
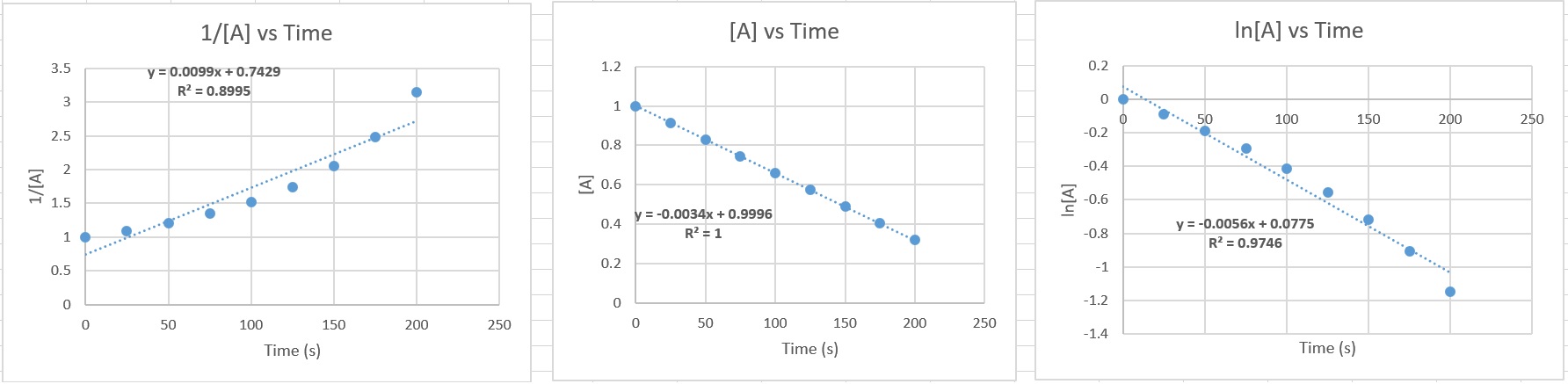
these questions 67.1 Refer to the graphs above and answer the following questions: a. In which graph does the data fit a straight line best? b. What is the reaction order in this example? c. What was the initial concentration of AB? i.e. [AB]0 d. What is the value of the reaction rate constant with units? e. Calculate the AB concentration at 230. seconds. f. Write out the reaction rate law for this reactant. 67.1 a. Third graph from left 1/[AB] vs time b. When the graph of 1/[AB] vs time is a straight line, the reaction is second order. c. 0.944 M d. 0.0225 M-1s-1 e. 0.160 M f. rate = k [AB]2 67.2 |
|
 |
|
| 67.2 Refer to the graphs above and answer the following questions: a. In which graph does the data fit a straight line best? b. What is the reaction order in this example? c. What was the initial concentration of A? i.e. [A]0 d. What is the value of the reaction rate constant with units? e. Calculate the AB concentration at 250. seconds. f. Write out the reaction rate law for this reactant. 67.3 The half life, t½ , is the time required for exactly ½ of the original material to consumed. In the table above, the expressions are given for zeroth, first and second order reactions. Note that only the second order half life depends on the initial concentration. In all other cases, the half life is independent of concentration. a. The first order nuclear decay of U-238 has a rate constant of k = 1.551 x 10-10 yr-1 What is the half life for U-238 in years? b. The first order nuclear decay of carbon-14 has a rate constant of 1.2097 x 10-4 yr-1 What is the half life for C-14 in years? 67.4 Starting with 5 grams of a radioactive element (first order decay), how much remains after ..... a. 1 half life b. 2 half lives c. 3 half lives d. 4 half lives e. 10 half lives. 67.5 In the first order reaction D --> products, it is found that 90% of the original amount of reactant D decomposes in 140. minutes. Find the half life of the decomposition reaction. 67.6 The first-order decay of radon has a half-life of 3.823 days. How many grams of radon remain after 7.22 days if the sample initially weighs 250.0 grams? 67.7 Protactinium-231 has a half life of 3.24 x 104 years. How long will it take for 31% of the original sample to decay? Answers: Click and drag in the space below 67.1 a. Third graph from left 1/[AB] vs time b. When the graph of 1/[AB] vs time is a straight line, the reaction is second order. c. 0.944 M d. 0.0225 M-1s-1 e. 0.160 M f. rate = k [AB]2 67.2 a. Second graph from left [A] vs time b. Zeroth order c. 0.9996 M (~1.0 M) d. 0.0034 M/s e. 0.1496 M f. rate = k [A]0 = 0.0034 M/s [A]0 67.3 a. For U-238 t½ = 4.47 billion years b. For C-14 t½ = 5729.9 years 67.4 a. First half life = ½ x 5 grams = 2.5 grams b. Second half life = ½ x ½ x 5 grams = 1.25 grams c. Third half life = ½ x ½ x ½ x 5 grams = 0.625 grams d. Fourth half life = (½)4 x 5 gram = 0.3125 grams e. Tenth half life = (½)10 x 5 gram = 0.004883 grams 67.5 t½ = 42.14 minutes 67.6 67.5 grams remain .... 182.5 grams have decomposed. 67.7 1.73 x 104 years |
|
| Thursday July 23, 2020 (Part 2) Reaction Mechanisms I and the RATE LIMITING STEP (RLS) |
|
| Textbook references 14.6: Reaction Mechanisms Additional: Reaction Mechanisms |
Course Lectures 6.1 pdf Video Type 1 Rxn Mechanisms |
| The Rate Limiting Step |
The Rate Limiting Step |
| Objectives 1. Define the rate limiting step and identify rate limiting steps in real life 2. Identify the rate limiting step in type I reaction mechanisms 3. Define "elementary reaction" |
Type 1 Rxn Mechanisms |
4. Know what factors characterize an elementary reaction (uni or bimolecular) 5. Combine elementary reactions to determine the net reaction for the mechanism 6. Identify reaction intermediates in a reaction mechanism 7. Write the mechanism rate equation using the rate limiting step. |
|
| Answer
these questions 68.1 "Elementary reactions" are the individual steps that when taken together reproduce the overall reaction as the "net reaction." Why are uni-molecular and bi-molecular reactions allowable whereas tri-molecular reactions are not? 68.2 Examine the following two - step Type I reaction mechanism: Step 1 CH4(g) + Cl2(g) ⟶ CH3(g) + HCl(g) + Cl −(g) (Slow) Step 2 CH3(g) + Cl2(g) ⟶ CH3Cl(g) + Cl −(g) ( Fast) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? 68.3 Examine the following four-step Type I reaction mechanism: Step 1: HBr + O2 → HOOBr (Slow) Step 2: HOOBr + HBr → 2 HOBr (Fast ) Step 3: HOBr + HBr → H2O + Br2 (Fast ) Step 4: HOBr + HBr → H2O + Br2 (Fast ) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? 68.4 Examine the following four-step Type I reaction mechanism: Step 1: H2O2 → H2O + O (slow) Step 2: O + CF2Cl2 → ClO + CF2Cl (fast) Step 3: ClO + O3 → Cl + 2O2 (fast) Step 4: Cl + CF2Cl → CF2Cl2 (fast) a. What is the net reaction for this mechanism? b. What is (are) the reaction intermediate(s)? c. Which step is the RLS? d. What is the reaction rate equation for the mechanism? 68.5 On the next exam, you'll be asked to describe a multi-step process from your life. You'll need to provide a list of the steps and then identify the RLS with reasons that explain why it's the rate limiting step. Answers: Click and drag in the space below 68.1 Tri-molecular reactions would require a collision between three atoms and/or molecules. The likelyhood of three particles colliding at exactly the same moment and with exactly the required orientation is low and therefore unlikely. 68.2 a. CH4(g) + 2Cl2(g) ⟶ CH3Cl(g) + HCl(g) + 2Cl −(g) b. CH3(g) c. Step 1 d. Rate = k[CH4][Cl2] 68.3 a. 4 HBr + O2 → 2 H2O + 2 Br2 b. HOOBr & HOBr c. Step 1 d. Rate = k[HBr][O2] 68.4 a. H2O2 + O3 → H2O + 2O2 b. O, ClO, CF2Cl, Cl c. Step 1 d. Rate = k[H2O2] 68.5 Looking forward to seeing what you come up with! :) |
|
| Wednesday July 22, 2020 (Part 1) Effective Collisions and Catalysts |
|
| Textbook references 14.5: The Effect of Temperature on Rxn Rate. 14.7: Catalysis |
Course Lecture 5.1 pdf Video Molecular Collisions 5.2 pdf Video Catalysts |
| Energy diagrams, Catalysts and Reaction
Mechanisms |
Catalyst Classes |
| Objectives 1. Identify forward and reverse activation energies from a reaction profile and use them to determine ΔHrxn and whether the reaction is exothermic or endothermic. 2. Inspect reactant and product molecular shapes and determine possible transition state (activated complex) arrangements. 3. Describe what is meant by an effective molecular collision and the factors that contribute to effective collisions. 4. Relate the magnitude of the frequency factor "A" in the Arrhenius equation to reactant collision orientations. 5. Describe what catalysts are and how they affect the rate of reaction on a molecular level. |
|
| Chemistry
takes place when atoms and molecules collide effectively. To be
an effective collision, the collision must satisfy two important
criteria: 1. The collisional energy must be greater than the activation energy Ea. 2. The reactant species must collide with the correct orientation to produce products. This last criterion is described by the frequency factor "A" in the Arrhenius equation:  |
|
As an example of a large "A" situation, consider the reaction below and at right: N(g) + O(g) → NO(g) Because both reactant atoms are roughly spherical (figure at right), it doesn't matter how they collide; only that they do. As long as their energies are greater than Ea, every collision is effective and produces products. In this case reactant orientation does not matter and A, kT and reaction rate are all larger. |
 |
As an example of a relatively smaller "A" situation, consider the following reaction: NO(g) + NO(g) → N2(g) + O2(g) Demonstrated below are two NO molecular collisions. To identify effective collisions from ineffective collisions, you must first look at the products to see what connections must be made. In this case we see that the two nitrogen atoms must connect with each other to form N2. Similarily, the two oxygen atoms must connect with each other to form O2. If, during the collision, the two N's and O's don't collide with eachother, the collision is ineffective. Referring to the diagram below, the first collision is ineffective as the N's and O's aren't lined up with each other when the molecules collide. In the second example, O's and N's contact each other forming what's called a transition state (also called an activated complex) which is positioned at the top of the activation energy hump. In the transition state, the N-N and O-O bonds are forming whilst the N-O bonds are breaking. When finished, the products go their separate ways. For this reaction, reactant molecule orientation does matter. Not all collisions have the right oreination to form products. Thus, A, kT and the reaction rate are smaller. |
|
 |
|
| Homework
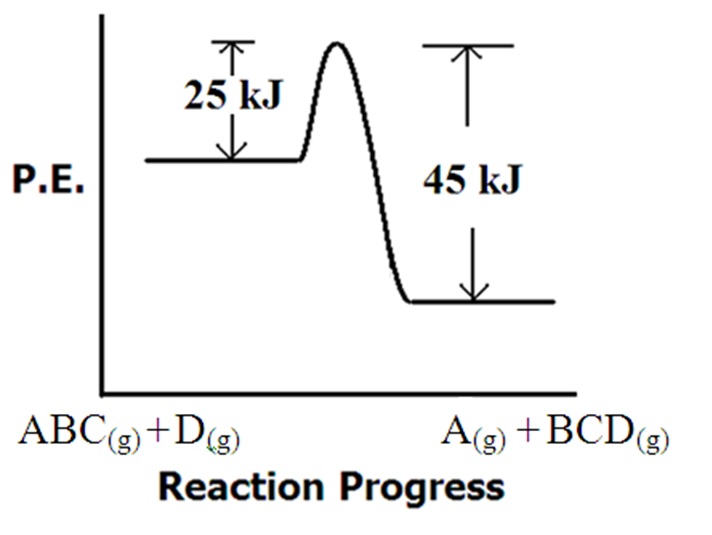
Questions Referring to the figure at right .... 65.1 a. What is the value of the forward activation energy? b. What is the value of the reverse activation energy c. What is the value of ΔH for the reaction and is it exothermic or endothermic? |
|
|
d. Will any collision between D and ABC produce a product? (For help on this look at the products that form and decide how much they depend on collisional orientation) e. Which of the following is the most likely transition state for this process?  |
|
| 65.2
Reaction #1 has a very large frequency factor "A" while Reaction
#2 has a very small frequency factor comparatively. All other factors being equal, which of these two reactions should have a faster reaction rate? 65.3 Reaction A has a very small frequency factor value while Reaction B has a very large frequency factor value. All other factors being equal, which of these two reactions is less affected by molecular orientation? 65.4 Catalysts speed up chemical reactions. What variable changes in the Arrhenius equation when a catalyst is present? |
|
65.5 a. Referring to the figure at right, what is the value of ΔHrxn? b. What is the activation energy of the catalyzed reaction? c. By how much is the activation energy reduced when a catalyst is present? d. Why does the presense of a catalyst lower the forward activation energy? |
 |
| 65.6 As we'll learn later, chemical reactions often occur in steps that together are considered a reaction mechanism. The introduction of a catalyst sometimes produces additional steps that occur behind the scenes which ultimately lower the activation energy and speed up the reaction. a. The figure at right shows the reaction profile both with and without catalyst. What is ΔH for the reaction? b. For the catalyzed reaction, how many steps take place behind the scenes? |
 |
| c. What are the forward Ea values for each of the catalysis steps? d. How much activation energy does the catalyzed reaction save in comparison to the uncatalyzed reaction? Answers: Click and drag in the space below 65.1 a. Eaf = 25 kJ b. Ear = 45 kJ c. ΔH = -20 kJ (exothermic) d. No. Refer to the reactants and products that appear along the x axis. Looking at the products, it's clear that "D" is attached to "C". Also, "A" is now separate. This will require bond formation between C & D and the A-B breaking. Consequently, when D collides with ABC, it'll be important for "D" to collide with the "C" end of the ABC molecule. e. "c" is the correct answer. It shows the A-B bond breaking and the C-D bond forming. 65.2 The rate of reaction is proportional to kT. kT is proportional to the frequency factor "A". Therefore, the rate of reaction is proportional to the frequency factor "A". All other factors being equal, the reaction with the larger frequency factor will be faster. 65.3 When molecules must collide in very specific ways, the frequency factor is smaller. When how the molecules collide isn't important, the frequency factor will be larger. Reaction B involves collisions that are less affected by molecular orientation. 65.4 Activation Energy Ea 65.5 a. + 12.5 kJ (endothermic) b. 32.3 kJ c. Activation energy lowered by 11.3 kJ d. Many reasons possible. The catalyst may introduce multiple steps to the process that have overall lower activation energies. Also the catalyst may direct the orientation of reactant species making the necessary bond breaking/making lower in energy. 65.6 a. - 56 kJ (exothermic) b. Two steps c. Ea1 = 26 kJ Ea2 = 21 kJ d. 32 kJ savings in activation energy. |
|
| Wednesday July 22, 2020 (Part 2) Integrated Rate Equations |
|
| Textbook references 14.4 The integrated Rate Law: The Dep. of Concentration on Time |
Course Lectures 4.4 pdf Video Integrated Rate Equations 4.5 pdf Video Integrated Rate Equation Example |
| Objectives 1. Identify correct integrated rate expression to be used in a problem 2. Given initial concentration and rate constant, calculate the final concentration at some other time. 3. Given the concentration and rate constant, determine the initial concentration. 4. Adapt the integrated rate laws for use in situations utilizing percentages. |

|
The integrated rate law equations are powerful mathematical tools that let us determine what remains of a reactant after some amount of time has passed. These equations are listed in the table above as "Integrated Rate Laws". To use the equations, you must know the "order" of the reaction of which there are three: Zeroth "0", First "1" Second "2". Knowing this lets you determine which of the three equations to use. The remaining variables are: [A]0 ...the initial concentration or amount of "A" initially present. [A]t ... the amount of "A" present at time "t" k ... the reaction rate constant t ... time (any unit is okay as long as its consistent with "k") Knowning any 3 of these variables, lets you solve for the 4th. |
|
| Answer
these questions. 66.1 Zeroth order rate behaviors are frequently observed in manufacturing where product is constructed from a finite supply of parts. Consider the following overly simplified construction of automobiles: 4 Wheels + 1 Body → 1 Automobile a. Assuming unlimited "Bodies", how many automobiles could be constructed from the 500. wheels stored in the factory? b. The rate constant "k" for this zeroth order process is + 3.75 wheels/hr-1. How many hours will it take for the entire supply of wheels to be exhausted? c. Assuming an 8 hour workday, how many days will the wheel supply last? d. How many wheels are left after 3.5 work days? e. How many days are required to use 60. wheels? f. How many days are required to use half of the available supply of wheels? 66.2 A liquid evaporates following zero order kinetics with k = 6.75 x 10-3 g s-1. How long in seconds does it take for 0.010 kg of the liquid to evaporate? 66.3 The decomposition of SO2Cl2 is first order in SO2Cl2 and has a rate constant of 1.42 x 10-4 s-1. a. If the initial concentration of SO2Cl2 is 1.50 M, what is the concentration after 60.0 seconds have passed? b. How much time (s) is required for the concentration of SO2Cl2 to reach 0.50 M? c. How much time (s) is required to use 1.33 M of the original 1.50 M SO2Cl2 ? 66.4 Radioactive decay is a first order process. Consider Uranium 238 (U-238): It decays via first order kinetics with a rate constant of k = 1.551 x 10-10 yr-1. a. How much of a 10.0 gram sample is left after one billion years? b. How much of a 10.0 gram sample decayed after one billion years? c. How much time (yr) is required for 30% of the sample to remain? d. How much time (yr) is required for 30% of the sample to be consumed? 66.5 Carbon 14 (C-14) decays via first order kinetics with a rate constant of k = 1.2097 x 10-4 yr-1 . a. How many years have passed if 30.% of the original sample remains? b. How many years have passed if 99% of the original sample has decayed? Answers: Click and drag in the space below 66.1 a. 125 complete cars b. 133.3 hours c. 16.67 days d. 105 wheels used ... 395 wheels left e. 2 days f. 8.33 days 66.2 1,481 seconds 66.3 a. 1.487 M b. 7,740 seconds c. 1.53 x 104 sec. 66.4 a. 8.56 grams remaining b. 1.44 grams decayed c. 7.762 billion years d. 2.299 billion years 66.5 a. 9,953 years b. 38,000 years |
|
| Tuesday July 21, 2020 (Part 1) The Rate Equation |
|
| Textbook references 14.3 The Rate Law: The Effect of Concentration on Reaction rate |
Course Lectures 4.3 pdf Video The Reaction Rate Equation |
| |
|
| Objectives 1. Provide the general form of a reaction rate equation 2. Write the reaction rate equation given reactant order information 3. Identify reactant and overall reaction order given the reaction rate equation 4. Predict reaction rate changes given the reaction rate equation and concentration 5. From concentration and rate data, determine the reaction rate constant 6. Use the reaction rate law and rate constant value to determine the rate of the reaction. |
|
Homework Questions 63.1 A reaction is known to be first order in A, second order in B and zeroth order in C. a. Write the reaction rate equation b. How does the reaction rate change if the concentration of A is doubled and the other concentrations held constant? c. How does the reaction rate change if the concentration of B is doubled and the other concentrations held constant? d. How does the reaction rate change if the concentration of C is doubled and the other concentrations held constant? e. How does the reaction rate change if the concentration of all reactants are tripled? 63.2 Given the rate equation, rate = k [X][Y]2, answer the following questions. a. What are the individual and overall reaction orders? b. What are the units associated with all quantities in the rate equation? c. An experiment is performed where [X]i = 0.35 M [Y]i = 0.75 M If the rate of the reaction is measured to be 0.125 M/s, what is the value for "k" (with units)? d. Determine the rate of reaction when [X]i = 0.165 M [Y]i = 1.88 M (Assume conditions identical to part "c") 63.3 The following contains experimental data for the following reaction. The data summarizes experiments designed to determine the reaction rate equation for the reaction. NH4+(aq) + NO2-(aq) → N2(g) + 2H2O(l) Exp [NH4+]i [NO2-]i RATE ------------------------------------------------------- 1 0.010 M 0.020 M 0.020 M/s 2 0.015 M 0.020 M 0.030 M/s 3 0.010 M 0.010 M 0.005 M/s a. Examine the data highlighted in BLUE and answer the following questions: i. How does the concentration of [NH4+]i compare between the two trials? ii. How does the concentration of [NO2-]i compare between the two trials? iii. How has the reaction rate changed comparing the two trials iv. What does this say about the reaction rate order for [NH4+] ? Exp [NH4+]i [NO2-]i RATE ------------------------------------------------------- 1 0.010 M 0.020 M 0.020 M/s 2 0.015 M 0.020 M 0.030 M/s 3 0.010 M 0.010 M 0.005 M/s b. Examine the data highlighted in RED and answer the following questions: i. How does the concentration of [NH4+]i compare between the two trials? ii. How does the concentration of [NO2-]i compare between the two trials? iii. How has the reaction rate changed comparing the two trials iv. What does this say about the reaction rate order for [NO2-] ? c. Use what you've learned from parts a and b to write out the reaction rate equation. d. Use any experimental trial to determine the value of the rate constant k (with units) e. Determine the initial reaction rate when [NH4+]i = 0.018 M and [NO2-]i = 0.013M 63.4 Rate data were obtained for the following reaction: A + 2B → C + 2D EXP [A]i [ B]i RATE 1 0.10 M 0.10 M 3.0 x 10-4 M/min 2 0.30 M 0.30 M 9.0 x 10-4 M/min 3. 0.10 M 0.30 M 3.0 x 10-4 M/min 4 0.20 M 0.40 M 6.0 x 10-4 M/min a. Determine the rate law for this reaction b. Determine the value of the rate constant (with units) for this reaction c. Determine the initial reaction rate when [A]i = 0.25M and [B]i = 0.35M 63.5 The following data were obtained for the chemical reaction: A + B → C + D EXP [A]i [ B]i RATE 1 0.040 M 0.040 M 9.60 x 10-6 M/sec 2 0.080 M 0.040 M 1.92 x 10-5 M/sec 3. 0.080 M 0.020 M 9.60 x 10-6 M/sec Determine the initial reaction rate when [A]i = 0.060 M and [B]i = 0.030M Answers: Click and drag in the space below 63.1 a. rate = k[A]1[B]2[C]0 = k[A][B]2 b. 2 X rate c. 22 X rate = 4 X rate d. 20 X rate = 1 X rate e. 31 X 32 X 30 = 27 X rate 63.2 a. First order in X. Second order in Y. Third order overall b. [X]: Molarity1 [Y]: Molarity2 rate: M/sec k: M-2s-1 c. 0.6349 (1/M2 )(1/sec) d. 0.3703 M/s 63.3 a. i. A 1.5 X increase from trial 1 to 2 ii. No change iii. A 1.5 X increase from trial 1 to 2 iv. Since the rate depends directly on the concentration of NH4+ , it is first order in NH4+ . b. i. Concentration is the same. ii. Concentration doubles X 2 iii. Rate quadruples X 4 or X 22 iv. Since the rate depends upon the square of the NO2- concentration, it is second order in NO2-, c. rate = k [NH4+] [NO2-]2 d. 5000 M-2s-1 e. 0.01521 M/s 63.4 a. rate = k [A] (First order in A and zeroth order in B) b. k = 0.0030 min-1 c. 0.00075 M/min 63.5 Reaction is first order in A and B k = 0.0060 M-1s-1 rate = 1.08 x 10-5 M/s |
|
| |
|
| Tuesday July 21, 2020 (Part 2) The Arrhenius equation Temperature and Activation Energy |
|
| Textbook references 14.5: The Effect of Temperature on Rxn Rate. |
Course Lectures 5.3 pdf Video Arrhenius Equation |
| Activation Energy |
Arrhenius Equation |
| Objectives 1. Describe how changing the reaction rate constant, kT, affects the rate of reaction. 2. Explain what activation energy is and how it affect the rate of reaction. 3. Describe how temperature affects molecular energies and how this relates to activation energy. |
|
| 4.
Explain how changes in temperature, activation energy, and frequency
affect the reaction rate constant, kT, determined via the Arrhenius Equation. 5. Given any three of the following variables: Temperature (T), Activation Energy (Ea), Frequency factor (A) or reaction rate constant (kT), determine the fourth using the Arrenius Equation. |
|
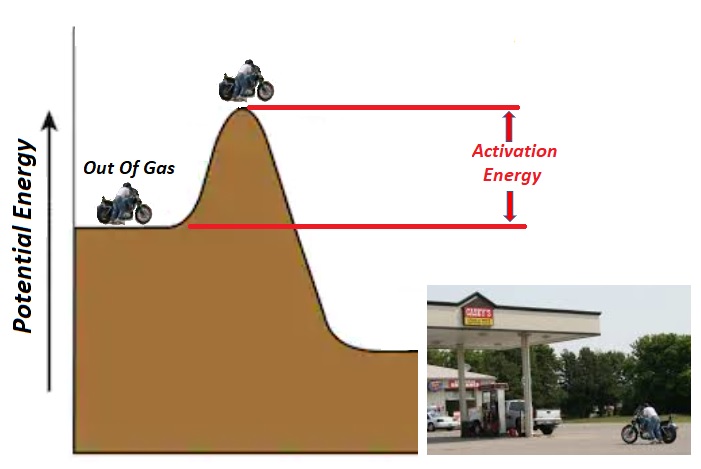
| Activation Energy: An anecdotal story Years ago, while out enjoying a motorcycle ride, I ran out of gas. The good news was that a gas station was near and at the bottom of a hill. The bad news was that I had to first push the bike up a hill before I'd be able to coast down to the gas station. It's a good thing I'd eaten breakfast because I'd need all my strength. The initial hill I'd have to overcome is known as "Activation Energy." |
|
 |
Chemical reactions take place when molecules collide. In the figure at left, the reactants A & B must collide with enough energy to reach the top of the hill (Activated Complex) before beginning their downhill slide into products (C & D). The energy required to reach the top is known as the forward activation energy (Eaf). Molecules that collide with energies less than Eaf, will not go over the top and thus won't form products. |
| The kinetic energies of molecules depend on
temperature. As temperatures go up, so do molecular kinetic
energies. However, at a specific temperature, molecular kinetic energies are not a single value. Rather, they are distributed over a range of values. The figure at right demonstrates how this distribution changes as temperatures are increased T1 < T2 < T3 < T4 Notice that as temperature is increased, the distribution extends and flattens. So, at higher temperatures, a greater fraction of reactants have kinetic energies greater than the activation energy Eaf. and these reactants, if they collide, can form products. |
 |
| Recall that the rate of
a reaction
depends on the
concentrations of reactants and the reaction rate constant kT. rate = kT
[A]x[B]y[C]z
However, the rate constant kT depends on temperature and activation energy. This dependence is given by the Arrhenius equation:  |
|
| Homework
Questions 64.1 Refering to the "Molecular Fraction vs. Molecular Energy" graph above, what are the approximate percentages of molecules possessing energies greater than the activation energy at each of the four temperatures? 64.2 Given Ea = 50.0 kJ and A = 5.56 x 105. a. Use the Arrhenius calculate kT for 288 K and 298 K. b. How does kT change for this 10o C temperature increase? c. How does the reaction rate change for this 10o temperature increase? d. Chemists often say that the rate of reaction doubles for every 10o temperature increase. Is that the case here? 64.3 The decomposition of ozone an important reaction in atmospheric chemistry studies. O3(g) → O2(g) + O(g) Given the frequency factor (A) of 4.36 x 1011 and activation energy of 93.1 kJ/mol, Calculate the reaction rate constant at 25oC and 35oC . By what factor does the reaction rate increase in this example? 64.4 Consider the hydrolysis of sucrose shown below: HCl C12H22O11 + H2O → C6H12O6 + C6H12O6 Sucrose D-Glucose D-Fructose Given that Ea = 108 kJ/mol , kT = 1.0 x 10-3 M-1s-1 at 37oC a. Determine the frequency factor "A" that using the Arrhenius equation. b. Once known, you can assume that Ea and A don't change for a reaction. Use what you now know about the reaction above to determine the reaction rate constant kT at 100oC 64.5 The reaction rate constant for a reaction, kT is known to be 0.44 s-1 at 25oC If the forward activation energy is known to be 245. kJ/mol, what is kT at 125 oC? How many times faster does the reaction go at this elevated temperature? 64.6 An alternative form of the Arrhenius equation (2 point formulation) eliminates the frequency factor A and relates pairs of k and T values:  Given that kT = 0.75 s-1 @ 25oC and that kT = 11.5 s-1 @ 75oC, use the Arrhenius equation above to determine the activation energy for the reaction. 64.7 The two point Arrenius equation above can be used to create what's known as an Arrhenius plot. 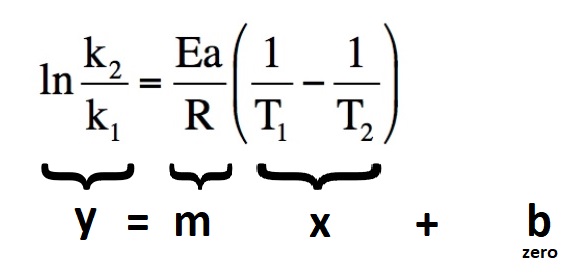 When (ln(k2/k1) is graphed versus (1/T1 - 1/T2), the straight line relationship let's us determine the activation energy from the slope: Consider the following Arrhenius plot constructed from the experimentally determined kT and temperature data. What is the activation energy for the reaction?  Click and drag below for correct answers. 64.1 The following percentages are visual approximations. Your results may vary somewhat. @ T1 (Orange) ~2% of all molecules have KE > Ea @ T2 (Orange + Red) ~ 20% of all molecules have KE > Ea @ T3 (Orange + Red + Green) ~ 30% of all molecules have KE > Ea @ T4 (Orange + Red + Green + Blue) ~45% of all molecules have KE > Ea 64.2 a. @ 288 K kT = 4.74 x 10-4 @ 298 K kT = 9.56 x 10-4 b. kT approximately doubles. c. Because the rate of reaction is proportional to kT, the reaction rate approximately doubles. d. Yes, while only an approximation, the reaction rate does seem to double for this 10o C temperature increase. 64.3 @ 298.15 K kT = 2.13 x 10-5 s-1 @ 308.15 K kT = 7.20 x 10-5s-1 3.4 X rate increase 64.4 a. A = 1.55 x 1015 b. @ 100oC kT = 1.18 64.5 @ 125oC kT = 2.66 x 1010 s-1 64.6 Ea = 47.12 kJ 64.7 Ea = 142.5 kJ |
|
| Monday July 20, 2020 (Part 1) Factors Affecting the Rate of a Reaction |
|
| Text book References 3.3: Factors Affecting Reaction Rates |
Course Lectures |
| Objectives 1. Describe on a molecular level how each of the following increases the rate of a chemical reaction: a. Temperature b. Concentration c. Surface area/mixing d. Catalyst |
|
| Homework
Questions 61.1 On a molecular level, why does an increase in temperature increase the speed of a reaction? 61.2 On a molecular level, why does an increase in concentration increase the speed of a reaction? 61.3 On a molecular level, why does an increase in surface area increase the speed of a reaction? 61.4 On a molecular level, why does the presense of a catalyst increase the speed of a reaction? 61.5 In Principles of Chemistry 1, there is an experiment where students dissolve solid I2 in methanol before reacting the solution with solid Zn metal to form ZnI. You might remember it as the "Empirical Formula of ZnI experiement". One of the post-lab questions asks about the role that methanol plays and frequently students make the incorrect claim that it's a "catalyst". Why is that answer incorrect and what role really does the methanol play in increasing the rate of reaction? 61.6 In the video above, how do each of the following relate to reaction rate? a. Reducing hallway space b. Reducing time between classes c. The "match maker" d. Banning groups of students Answers: Click and drag in the space below 61.1 Increasing the temperature of a reaction mixture increases the molecular kinetic energies. Thus, when molecules collide, they will strike with greater force making chemical changes (bond breaking and rearrangement) more likely. 61.2 Increasing reactant concentrations (decreasing volume/additional solute or increasing pressure) makes things more crowded and increases the chance that molecules will collide. 61.3 By breaking up reactants, we expose them and increase the chance that transformative collisions will occur. Again: More collisions = Faster reactions. 61.4 Catalysts coordinate the reactants making sure they come into contact in just the right way and produce chemical change. 61.5 In this experiment, the methanol is the "solvent". It dissolves the I2 making it possble for more I2/Zn contact. The methanol really increases the surface area or mixing of the reactants. Methanol is NOT a catalyst as it does nothing to direct the correct orientation of reactants. 61.6 a. Less space = more crowding = higher concentrations = faster reaction b. Less time = faster students = more energetic collisions = faster reaction c. Match maker = orient students for successful encounters = a catalyst = more effective molecular collisions = faster reaction. d. No student clusters = more surface area = more collisions = faster reaction. |
|
| Monday July 20, 2020 (Part 2) The Rate of a Chemical Reaction: Reactants, Products and Coefficients |
|
| Textbook references 14.2 Rate of a Chemical Reaction |
Course Lectures 4.1 pdf Video* Reaction Rates |
| Objectives 1. Determine the rate of a chemical reaction from graphical information and the balanced chemical equation 2. Express the rate of a chemical reaction in terms of reactants or products given the balanced chemical equation |
|
| Homework
Questions 62.1 The graph at right was obtained for this reaction: 2A(g) → B(g). A tangent is drawn to the point high- lighted by the black dot. The slope of this line is represents the rate of change of "A" at that point. (a.k.a. the "instantaeous rate of change of A". What is the slope of the line and what are its units? |
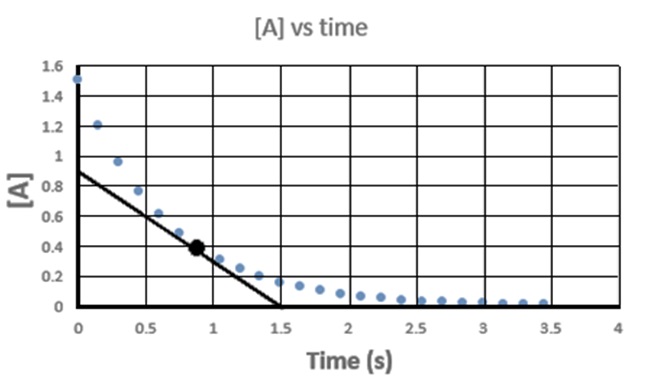 |
| 62.2
The slope of the line you determined in
62.1 is NOT the rate of this reaction for two reasons: i. The rate of the reaction is always reported as a positive number ii. The slope overestimates the rate since "A" is disappearing twice as fast as "B" is appearing. a. What is the rate of appearance of "B" in this case? b. What should be reported as the rate of this reaction? |
|
62.3 The graph at right was obtained for this reaction: 3A(g) + 2B(g) → C(g) + ½ D(g) Use the graph at right to determine the... a. The rates of change for all products and reactants. b. Rate of reaction |
 |
62.4 Consider the reaction N2(g) + 3 H2(g) → 2NH3(g) If the rate of formation of is measured to be NH3 is + 0.50 M/s, a. What are the rates of consumption for N2 and H2? b. What is the rate of reaction? 62.5 Consider the following reaction: 2 NO(g) + Cl2(g) → 2 NOCl(g) If Cl2(g) disappears at a rate of - 0.16 M/s, a. What is the production rate of NOCl? b. What is the rate of reaction? Answers: Click and drag in the space below 62.1 -0.60 M/s 62.2 a. + 0.30 M/s b. rate = 0.30 M/s (rate is always positive and independent of coefficients.) 62.3 a. RateA = - 1.018 x 10-3 M/s RateB = - 6.788 x 10-4 M/s RateC = + 3.394 x 10-4 M/s RateD = + 1.697 x 10-4 M/s b. Raterxn = 3.394 x 10-4 M/s 62.4 a. RateN2 = - 0.25 M/s RateH2 = - 0.75 M/s b. Raterxn = 0.25 M/s 62.5 RateNOCl2 = 0.32 M/s b. Raterxn = 0.16 M/s |
|